LUC示蹤細胞系底物使用方法和注意事項介紹
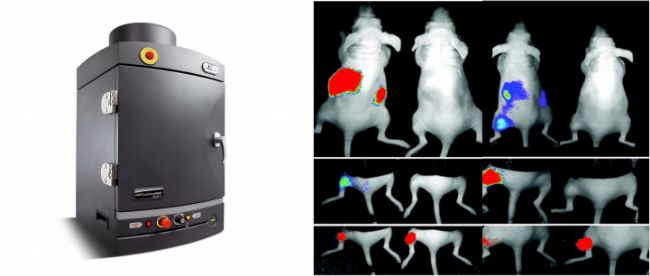
Materials
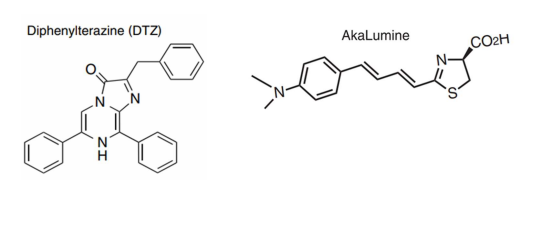
· Luciferase Substrate:
Luc1 Substrate (DTZ) (Meisen, Cat# CTCC-002-023)
Luc2 Substrate (AkaLumine-HCl) (Meisen, Cat# CTCC-002-024)
· DPBS (Dulbecco's Phosphate-Buffered Saline), without Ca2+ or Mg2+
· Syringe Filter, 0.2µm
Substrate Preparation
1. Thaw the substrates at room temperature and dissolve in DPBS (no calcium or magnesium) to the suggested final concentration.
→ Luc1 Substrate (DTZ): 3 mM DTZ solution containing 8% glycerol, 10% ethanol, 10% hydroxypropyl-β-cyclodextrin, and 35% PEG-400 in sterile water;
→ Luc2 Substrate (AkaLumine-HCl): 15 mM AkaLumine-HCl in saline;
2. Inoculate lab animal according to one of the various methods detailed below. (Luc1 and Luc2 substrates are typically administered either intraperitoneally or intravenously.)
→ Suggested starting dose: 100µl of prepared substrates for a 20g mouse.
3. Wait 10-20 minutes before imaging for maximum luciferase signal plateau.
4. A kinetic study of luciferase should be performed for each animal model to determine the peak signal time and plateau phase.
Determining the Kinetic Curve
The kinetics of tissue biodistribution may be different for each animal model and experimental design. We recommend creating a kinetic curve for each system prior to conducting the experiment in order to determine the peak signal time for imaging the animal model after the substrate injection.
Determining the Kinetic Curve
The kinetics of tissue biodistribution may be different for each animal model and experimental design. We recommend creating a kinetic curve for each system prior to conducting the experiment in order to determine the peak signal time for imaging the animal model after the substrate injection.
To generate a kinetic curve for luciferase activity in your model:
1. Inject the substrate via one of the proposed methods listed below (Intraperitoneal (IP) or Intravenous (IV)). If you need to sedate the animals before injection, be aware that it may slightly extend the kinetics (peak luciferase expression time). The biodistribution of Luc substrate may also be different depending on the route of administration.
2. For IP Injection
a. If you are able to inject into animals which are still awake, wait three minutes, then sedate by your method of choice (gas or injectable anesthesia).
b. Place sedated animals in imaging chamber and take the first image approximately five minutes post Luc substrate injection.
b. Place sedated animals in imaging chamber and take the first image approximately five minutes post Luc substrate injection.
3. For IV Injection
a. Immediately sedate the animal ( if not already sedated ) by your method of choice (gas or injectable anesthesia).
b. Place sedated animals in imaging chamber and take the first image within first two minutes post substrate injection
4. Continue to take images every 5-10 minutes up to about 40-60 minutes for IP injected models (for IV injected models: image every 1-5 minutes up to 20-30 minutes) to generate a kinetic curve for luciferase expression in your model.
· Most injectable anesthesia will last 20-30 minutes and might require an additional dose for a full kinetic curve study!)
5. Choose the best time point to image your model from your kinetic curve. Many models reach their peak signal time approximately 10-20 minutes post IP injection and 2-5 minutes post IV injection.
Intraperitoneal Injection (IP) in mice

1. First locate the point of entry for the needle
2. Draw an imaginary line across the abdomen just above the knees (see image above).
3. The needle should be inserted along this line on the animal's right side and close to the midline. (The point of entry is cranial to and slightly medial of the last nipple in a female mouse.)
4. Inserting the needle on the mouse's right side avoiding the cecum, which is a large fluid-filled organ on the left side of the abdomen. The small intestines (on the right side) are less likely to be punctured by the needle.
5. Inserting the needle too far caudally or laterally from the insertion point shown above would risk making an injection into the rear leg which would injure the muscle tissue.
6. To perform an IP injection, the mouse must be well restrained so that it cannot move during the procedure. This avoids traumatizing the organs once the needle has entered the abdomen.
7. Restrain the mouse and tilt so that the head is facing downward and its abdomen is
exposed.
8. Thoroughly disinfect the injection site with 70% ethanol. 9. We recommend using
a 25-27-gauge needle for IP injections. Insert the needle into the abdomen at about
a 30-degree angle.
Intravenous Injection (IV) in mice
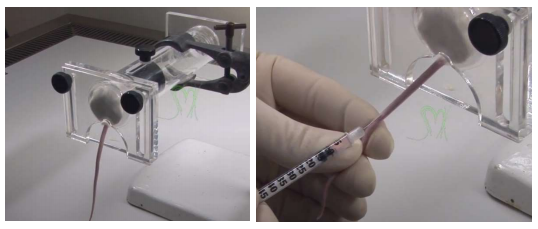
Restrain the mouse with physical or chemical restraint. Rotate the tail slightly to visualize vein. Disinfect injection site with 70% ethanol and insert the needle (27-30 gauge) into the vein at a slight angle. You will not be able to aspirate, instead inject slowly and watch for clearing of the lumen. Incorrect positioning will result in a slight bulge in the tail. If this occurs, remove needle and repeat process proximal to previous site. Upon completion, remove needle and apply pressure to injection site. Record the injection time to determine peak signal time.
標簽:
LUC示蹤細胞系底物
Copyright(C) 1998-2025 生物器材網 電話:021-64166852;13621656896 E-mail:info@bio-equip.com